Abstract
Introduction: Mantle cell lymphoma (MCL) is a non-Hodgkin's lymphoma (NHL) subtype with a unique clinical behavior which shares clinical characteristics of both aggressive and indolent NHL. While the majority of patients (pts) with MCL achieve complete remission (CR) with frontline therapy, a minority of pts experience primary treatment failure (PTF) defined herein as either progressive disease during frontline therapy, termed primary progression (PP), best response to frontline therapy of partial remission or stable disease, termed residual disease (RD), or relapse within 6 months of the end of therapy, termed very early relapse (VER). In a retrospective cohort of diffuse large B cell lymphoma pts, inferior outcomes were reported in pts with PTF and risk factors were indentified which are associated with decreased 2 year overall survival (OS) (Costa et al, Am J Hematol 2017 92: 161-170). In follicular lymphoma, early relapse (ER) within 24 months of initial chemo-immunotherapy (CI) has been associated with inferior outcomes (Casulo et al, J Clin Oncol 2015 33: 2516-22). To our knowledge, no study has described outcomes specific to MCL pts with PTF, and ER has been described only in the context of ER following autologous stem cell transplant (ASCT). In this retrospective study, we identified pts with MCL treated at our institution with either PTF or ER and described outcomes.
Methods: Eligible pts were ≥ 18 years with PP, RD, VER, or ER as defined above. After obtaining IRB approval, we identified pts treated at OSU with MCL diagnosed between 2002 and 2017 who met inclusion criteria. Patients receiving less than 2 cycles of treatment were excluded, except in cases of disease progression after cycle 1 of treatment. Baseline patient characteristics, salvage treatments received, response, and date of death or last follow-up were obtained from medical records. Baseline characteristics and salvage outcomes were compared using Wilcoxon signed rank test or Fisher's exact test. OS was defined as time from first progression to death, censoring pts alive at last follow-up, and estimated using the Kaplan Meier method.
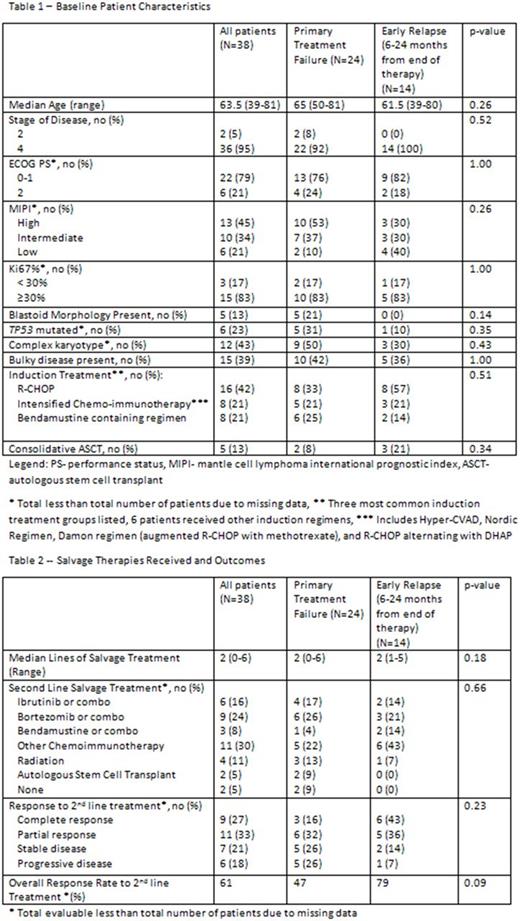
Results: 38 pts met the inclusion criteria; 24 with PTF and 14 with ER after 6 months. PTF was due to PP in 7 pts, RD in 11, and VER in 6. Among pts with PTF, 21% had blastoid morphology, cytogenetics when obtained at diagnosis showed 50% with complex karyotype and 31% with mutations in TP53, and 53% had a high risk MIPI score at diagnosis. Among pts with ER, the majority were low or intermediate risk by MIPI (70%), and fewer pts had blastoid morphology, complex karyotype, or mutations in TP53 compared with PTF, but none of these differences showed statistical significance (Table 1). Ki67 when performed was ≥ 30% in 83% of pts with PTF and ER. Estimated median OS from first disease progression was 13 months (95% CI: 5-23) for pts with PTF vs 29 months (95% CI: 10-99) for pts with ER with a trend towards statistical significance (p=0.08). Two year OS was 27% (95% CI 10-48%) for pts with PTF vs 54% (95% CI: 25-76%) for ER. Salvage therapies and response to treatment are summarized in Table 2. The median number of lines of salvage therapy was 2. The overall response rate (ORR) to second line therapy was 47% including 16% CR among pts with PTF compared with 79% ORR and 43% CR for pts with ER (p=0.09 for ORR). Among all pts, second line therapy included either single agent or combination therapy with bortezomib in 9 pts (ORR 44%), ibrutinib in 6 pts (ORR 60%), bendamustine in 3 pts (ORR 67%), other CI regimen in 11 pts (ORR 60%), and radiation in 4 pts (ORR 100%) with no statistically significant association between treatment and response. 45% of pts received ibrutinib at any point during salvage therapy. Two pts with PTF underwent salvage ASCT and 4 pts went on to allogeneic transplant (2 PTF, 2 ER).
Conclusions: In this single center retrospective study, primary refractory MCL was associated with poor clinical outcomes. Pts with ER between 6 and 24 months from end of therapy may have better outcomes in comparison to patients with PTF, but conclusions are limited due to the sample size. The median OS from diagnosis for pts with ER compares unfavorably to historical averages for newly diagnosed MCL. This study included pts treated prior to the ibrutinib era, and observations should be taken in this context. Further study is warranted to better define outcomes for MCL pts with PTF and those with ER, and novel treatment approaches are needed.
Christian: Janssen: Research Funding; Immunomedics: Research Funding; Merck & Co., Inc.: Research Funding; Pharmacyclics: Research Funding; BMS: Research Funding; Acerta: Research Funding; Seattle-Genetics: Research Funding; Genentech: Research Funding; Roche: Research Funding; Celgene: Research Funding. Maddocks: Novartis: Research Funding; Merck: Research Funding; BMS: Research Funding; Pharmacylics: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.